Desalination is the method of removing minerals like salt from ocean water to make it drinkable. It works one of two ways: by mimicking the sun and evaporating the water or forcing the water through a filter to separate the salt.
Either way, the result is fresh water safe for consumption.
What Is Desalination?
Desalination is the process that occurs to remove salt from salt water, making it drinkable for humans. 96.5% of the earth’s water is salty, making it impossible for humans or most animals to drink.

The other 3.5% is available for human consumption, but much of it remains trapped in polar ice caps or huge lakes. It’s challenging to move water around the world, and many places need fresh water every year.
Fresh water is an essential supplement to life. No one can survive without it for more than a few days, and dehydration is one of the most significant health issues on the planet. However, there is much more saltwater than fresh water, and the human body can’t function on salt water.
The fresh water crisis is a humanitarian issue and something that desalination can help solve. In reality, the process is more expensive than it is worth at times, and it becomes difficult to justify creating more desalination plants.
Ocean water only contains 2.5% salt, but that’s enough to dehydrate a human and cause eventual death if it’s consumed instead of fresh water. The human body is 70% fresh water and will die in about three days without drinking any clean water.
While access to the existing fresh water is an issue, the more significant problem is what humans will do if we ever run out. This fear is where desalination comes in as a potential solution.
There is nearly unlimited water in the ocean – and although it costs money, desalination might be the future of a planet with dwindling fresh water resources.
How Does Desalination Work?
Desalination works by separating the salt molecules from the water molecules. Because salt is heavier than water, the most common method is to evaporate the water molecules using the heat of the sun or another source. This system is called solar desalination.

Solar desalination is nature’s way of taking salt away from water. This process naturally occurs in the rain cycle, when the sun’s heat pulls water from oceans and lakes into clouds for rain. The salt gets left behind, and natural rain is fresh water.
Of course, the process doesn’t only happen in nature–scientists have successfully copied it for massive desalination plants worldwide. What started as simple, sun-powered bubbles have become huge plants dedicated to creating clean water out of salty.
Desalination has existed for thousands of years. Many ancient civilizations used solar desalination to procure fresh drinking water while sailing on the ocean. The process hasn’t changed too much–just the size and capacity of the mechanisms used for desalination.
What Are the Common Desalination Methods?
There are two main methods for desalination: solar desalination and reverse osmosis desalination. While solar desalination depends on the power of the sun and the position of the water, reverse osmosis forces salt water through a filter membrane to produce fresh water.
Solar desalination is the most common method and copies the natural rain cycle. Scientists can separate the salt molecules from the water by heating and evaporating salt water and creating clean water.
Usually, solar desalination gets completed with flash-evaporation. A flash of heat is applied to the water, and it turns to gas, leaving most of the salt behind. The gas is then trapped in a new container and allowed to cool down.
This process must repeat a few times to desalinate the water thoroughly. Otherwise, small salt molecules will remain, and the water will not be drinkable. Flash evaporation is similar to vapor compression, which also takes the gasses to a different container to separate the steam from salt.
Solar desalination can also occur through a distillation process, which involves heating the water through multiple filters until only the cleanest, salt-free water is available. This method is used for fresh water as well to ensure clean water for scientists and chemists.
Each of these methods releases the salt from the water and makes it potable. However, neither of them is inexpensive. The growing need for water is at odds with the massive amounts of money and energy necessary to run a desalination plant.
How Is Reverse Osmosis Used in Desalination?
It’s essential to know the phenomenon of osmosis to fully understand the process of desalination through reverse osmosis.
With osmosis, two different liquid forms will mix and even their density out, even with a semi-permeable membrane. The heavier and lighter liquids will become evenly distributed.
Osmosis is a naturally occurring process and has been studied extensively in science. However, reverse osmosis (RO) is a human occurrence when a single liquid is forced through a membrane to separate the heavier particles from the lighter ones.
For this to happen, pressure needs to be applied to counteract the osmotic pressure. The process removes chemical and biological species from the salt water, such as minerals and bacteria.
In the case of salt water, reverse osmosis works by forcing the salt water through a semi-permeable membrane. When it’s pulled through, the heavier salt particles remain on one side of the membrane while the lighter water molecules cross through.
Once the process is complete, the salt and water are effectively separated.
How Efficient Is Reverse Osmosis in Desalination?
Reverse osmosis is the most recent development in desalination science and the most efficient version. Instead of having to filter the water repeatedly or reboil it to vaporize the clean water, reverse osmosis only pushes water through once.
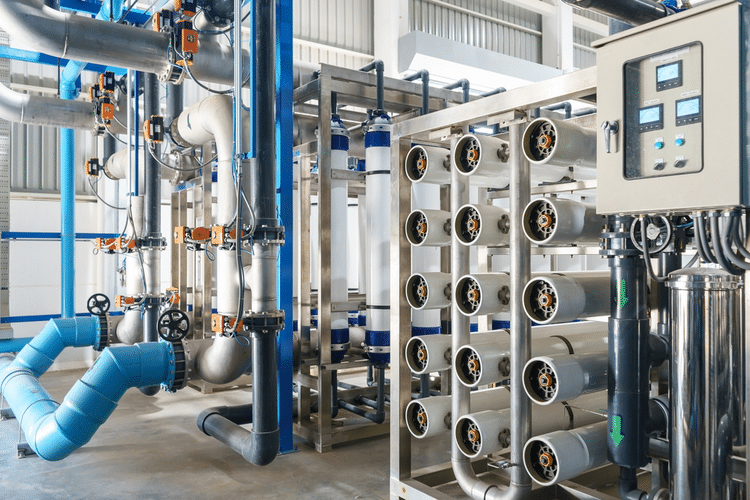
Unlike many dishwashers, washing machines, and toilets, reverse osmosis does not waste every gallon of water it uses. The entire process does require energy, but the energy gets almost fully recovered by the reverse osmosis itself.
The big downside of reverse osmosis desalination is that it takes about three gallons of salt water to make a gallon of osmosed water.
While this amount may seem excessive, the reverse osmosis system separates the minerals from the water through the semi-permeable membrane. Although it means that less fresh water gets produced through the process, the remainder of the salt water.
Why Is Desalination So Expensive?
If desalination can get more fresh water into the poorest nations in the world, why aren’t there more desalination plants around the country? The short answer is money. Desalination is expensive, and it’s more than just a startup cost: the plants cost money to build and run.
These costs cover the energy put into finding water and cleaning it, the costs of the building itself, and near-constant maintenance. After all, water is one of the biggest eroders in the world, and any building with a constant flow of water will need to be of robust material.
How Much Does a Desalination Plant Cost?
The cost of a desalination plant depends on the size of the plant. A smaller, 2.5 MGD (million gallons per day) will cost more than $32 million to build. However, a larger plant that can clean up to 100 million gallons per day will cost at least $700 million.
For a technology that is relatively new and not as efficient as we would like, it’s difficult to convince government officials and local benefactors to fund new desalination plants.
However, as science Kind Water Systemslves, prices might soon change.
Hi Scott
Wouldn’t it be better to spend $700 million on a water plant than billions of dollars on a high speed rail that is also going to have massive maintenance costs? Just a thought!
Thank You for your work!
MayGodBless
Hi Ted, thank you for reading our site. I know your question is more of a rhetorical one 😉 Let’s just say that water just continues to become even a more critical resource for much of the world and it sure makes sense to continue to develop our technology and capabilities around being able to deliver it where we need as a society. We know that through further development and experience, technology can become more effective and less costly. So, it’s a eminently worthwhile endeavor in my view.
Would it be possible to simply build large salt water reservoirs in flood plain areas to simply increase evaporation and thus precipitation. It seems as if filling the Saltin Sea and Great Salt Lake back to capacity with Salt Water would solve or alleviate a lot of issues.
Hi Paul, it’s a very interesting question and one that I’ve heard asked before and have contemplated myself. While I think that it’s certainly possible to have the dynamic you mention (pump water into areas that offer easy evaporative potential to help resultant precipitation later), I believe there would be many variable involved that would result in things likely not taking place exactly as desired. First and foremost from an economic perspective to even consider such a project, the case needs to be made for how salt water would be able to be brought to the area and the economic impact of the resultant precipitation. Next, in terms of feasibility and given how complex weather dynamics are, what is the probability that the precipitation will actually occur, what amount would be expected, and also where is that precipitation even likely to occur? Finally, there would need to be careful analysis of the environmental aspects of the entire operation, not the least of which is the introduction of a much greater amount of salt that would be introduced to the local environment after evaporation takes place. It’s a great question and one that elicits much interesting thought experiments. We would love to see if research has been done on this concept and what the results have shown.
Hello Scott,
Interesting article. Just a half baked thought but I was browsing to see if there might be a potential to use electrolysis to produce and store green hydrogen from seawater, then reconstitute into electricity and fresh water. I’m sure it has occurred to others as it would be a good fit for offshore wind energy storage. Probably not cost effective but any idea if it could become viable?
Hi Bob, thanks for your ideas here. I will admit that much of this is new to me, but you’ve inspired me to do some research in this area and understand these concepts. Let’s see where it leads!